The SARS-CoV-2 super strain, Omicron, has rapidly spread around the globe and gradually taken the place of previous Variants of Concern (VOCs) since first detected in last November. The Omicron variant bears more than 30 mutations in the Spike protein, allowing it to evade immunity from most therapeutic neutralizing antibodies and some vaccines. Therefore, the development of a potent neutralizing antibody against Omicron is of great clinical significance.
Recently Yongqun Zhu's Lab published a paper entitled 35B5 antibody potently neutralizes SARS-CoV-2 Omicron by disrupting the N-glycan switch via a conserved Spike epitope in Cell Host & Microbe, in collaboration with Kai Deng's Lab at Sun Yat-sen University, Zhiwei Chen's Lab at the University of Hong Kong, and Lilin Ye's Lab at the Third Military Medical University. The paper reported that the pan-neutralizing human monoclonal antibody (mAb) 35B5 of COVID-19 VOCs is capable of potently neutralizing Omicron and revealed its novel mechanism of action.

The team found that the receptor-binding domain (RBD)-targeting mAb 35B5 not only potently neutralizes previous VOCs, but also exhibits nanomolar level neutralizing efficacy to the Omicron variant. In order to study the structural basis for the immune escape of Omicron and the potent neutralization mechanism of 35B5, researchers analyzed the cryo-EM structures of the Omicron Spike extracellular domain (S-ECD) and the 35B5 Fab complex, and found that the Omicron S trimer exhibits tighter structural packing and higher thermostability. Further structural analysis revealed that the Omicron RBD is characterized by 15 mutation sites that induce significant structural changes of the mutant residues and antigenic shifts in RBD; in addition, Omicron NTD has 8 mutation sites, among which G142D and L212I are located on the surface, generating antigenic shifts of NTD.
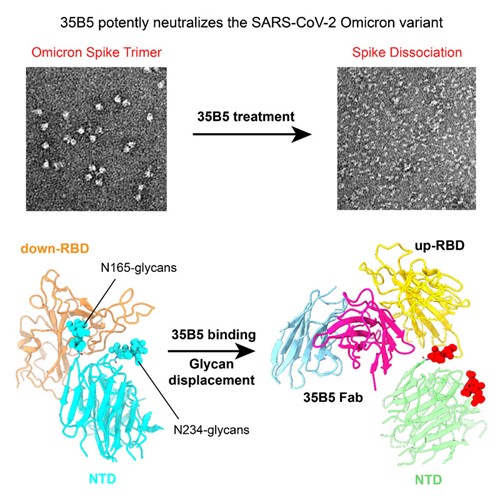
However, why these changes do not impact targeting of 35B5 to Omicron? Dr. Zhu and his collaborators found that the epitope of 35B5 on RBD is invariant among different VOCs, and the highly conserved epitope provides the molecular basis for 35B5 targeting the RBD of Omicron and other VOCs. Then the researchers analyzed why 35B5 epitope is so conservative. Results suggested that the epitopic residues of 35B5 are not only critical for the RBD structural integrity and ACE2 binding, but also participates in controlling the conformational dynamics of RBD. Transition from the “down” state to the “up” state of RBD is required for ACE2 recognition. The N-glycans at the residues N165 and N234 in NTD clamp the two sides of RBD, respectively, and function as a switch to control the conformational transition. And the N165-glycans, which interact extensively with the shallow pockets formed by the conserved epitopic residues Y351, T470, F490, and L452, play the key role in the closed conformation maintenance of the down-RBD. Upon 35B5 binding to S, the N165-glycans are displaced from its binding shallow pocket in the up-RBD and the N234-glycans are also completely released into the solvent, which opens the glycan switch. The displacement and dysfunction of the glycan switch at N165 and N234 of NTD by 35B5 likely lead to the unstable “up” states of RBD, which finally causes the dissociation of the S trimer. The glycan displacement action of 35B5 represents an unprecedented neutralization action of mAbs against SARS-CoV-2 (as shown in the above figure). This work shows that the potent neutralization efficacy against all VOCs and high antigenic-shift tolerance of 35B5 make it a good candidate in clinical therapy.
To access the article, please visit https://doi.org/10.1016/j.chom.2022.03.035
About Professor Yongqun Zhu
Professor Zhu graduated from Institute of Biophysics, Chinese Academy of Sciences in 2007 and became a professor in Life Sciences Institute, Zhejiang University from 2012. He is currently a Qiushi Professor and is an adjunct Professor of Shanghai Institute for Advanced Study of Zhejiang University (SIAS).
Prof. Zhu and his lab combine approached from microbiology, structural biology, biochemistry and cell biology to study the molecular mechanisms of bacterial-host interactions, and mainly focus on:
l Host modulation by the effector proteins from bacterial pathogens
l Commensal bacteria-host interactions
l Structural basis of bacterial-host interplay.
Since 2012, Prof. Zhu has published about 20 papers in high-profile journals, including Science, Cell, Molecular Cell, Nature Microbiology and PNAS, and revealed several new mechanisms in bacterial pathogenesis, including assembly of the bacterial flagellar motor and long-chain fatty-acylation modification. He has been awarded with the National Outstanding Youth Fund, the 16th China Youth Science and Technology Award and the Tanjiazhen prize.
This article was first posted from Prof.Zhu's lab.
About SIAS
Shanghai Institute for Advanced Study of Zhejiang University (SIAS) is a jointly launched new institution of research and development by Shanghai Municipal Government and Zhejiang University in June, 2020. The platform represents an intersection of technology and economic development, serving as a market leading trail blazer to cultivate a novel community for innovation amongst enterprises.
SIAS is seeking top talents working on the frontiers of computational sciences who can envision and actualize a research program that will bring out new solutions to areas include, but not limited to, Artificial Intelligence, Computational Biology, Computational Engineering and Fintech.

