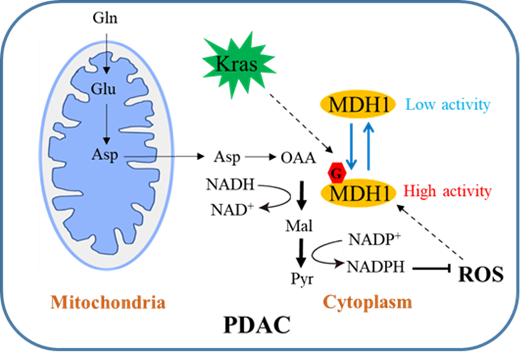
Pancreatic cancer is a malignant tumour of the digestive system, with only about 10% of patients having a five-year survival rate after diagnosis. Altered metabolism is one of the main characteristics of tumour cells. Tumour cells are metabolically reprogrammed to produce the substances, energy, and redox forces necessary for rapid proliferation. Cells from pancreatic ductal adenocarcinomas (PDAC, the most common pathological form of pancreatic cancer) activated by the oncogene Kras are highly dependent on a specific glutamine (Gln) catabolic pathway. In this pathway, Gln is first converted to aspartate (Asp), then converted to oxaloacetate (OAA) by glutamate transaminase (GOT1), which in turn is converted to malate (Malate) by malate dehydrogenase (MDH1). MDH1 is oxidised by malic enzyme (ME1) to pyruvate and reduced malate to pyruvate and reduced coenzyme II (NADPH), which provides the reducing equivalents to maintain intracellular redox balance. This metabolic pathway is critical for PDAC cell proliferation. Therefore, a deeper understanding of the regulatory mechanisms of this metabolic pathway may help to find new ideas and targets for the clinical treatment of PDAC. On July 25th, 2022, Professor Ruhong Zhou from Shanghai Institute for Advanced Study, Institute of Quantitative Biology, Zhejiang University, and Professor Wen Yi from the College of Life Sciences, Zhejiang University collaborated on an article in Nature Chemical Biology titled O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1. The work reveals a novel mechanism by which O-GlcNAc glycosylation regulates PDAC cell growth and explains the molecular mechanism by which glycosylation enhances MDH1 activity. This discovery has potential implications for pancreatic cancer drug development.

The O-GlcNAc glycosylation modification is a post-translational modification by the covalent attachment of N-acetylglucosamine to the serine (Ser) or threonine (Thr) hydroxyl group of the protein in the form of a β-glycosidic bond. This modification is a highly dynamic modification that changes rapidly in response to intracellular nutritional status and extracellular stimuli. The modification is widely found in intracellular proteins and regulates important biological processes such as gene transcription, signal transduction, protein synthesis, and metabolic reprogramming. Previous studies have shown that Kras-activating mutations promote O-GlcNAc glycosylation in cells and that abnormal levels of O-GlcNAc glycosylation are closely associated with the development of PDAC. However, the molecular mechanisms by which O-GlcNAc glycosylation regulates PDAC proliferation and tumorigenesis require further investigation. Professor Wen's group found that the expression of glycosyltransferase OGT was significantly increased in tumour tissues of pancreatic cancer patients, and that knockdown of OGT significantly inhibited Gln metabolism in PDAC cells, suppressing PDAC cell proliferation. Further studies revealed that O-GlcNAc modification promoted PDAC cell proliferation by regulating MDH1, a key enzyme in the Gln metabolic pathway. Using high-resolution mass spectrometry in combination with point mutagenesis, they identified position Ser189 as the site of glycosylation modification of MDH1. In vitro enzyme activity assays showed that glycosylation at the Ser189 position of MDH1 increased its enzymatic activity. Targeted metabolomics assays confirmed that MDH1 glycosylation promotes Gln metabolism and NADPH production. Moreover, MDH1 is involved in the intracellular malate-aspartate shuttle pathway that coordinates glycolysis and mitochondrial respiration, and MDH1 glycosylation leads to efficient NADH regeneration, thereby promoting mitochondrial respiration. In vivo tumour assays in mice and analysis of clinical samples also confirmed the promotion of PDAC growth by glycosylation modification of MDH1 position Ser189.
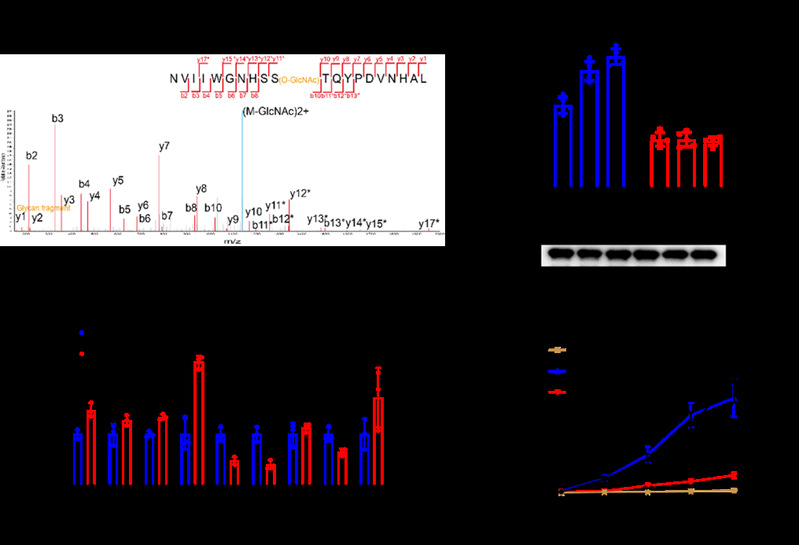
Mapping the site of O-GlcNAcylation on MDH1 using mass spectrometry and analysis and quantification of glycosylation levels on MDH1 using various site-directed mutants expressed in 293T cells (n = 3 independent assays).
To better understand the molecular mechanism by which O-GlcNAcylation enhances MDH1 protease activity, the authors investigated the kinetic behaviour of the MDH1 monomer-substrate complex (NADH, MAK) in both the glycosylated and nonglycosylated forms of Ser189 using molecular dynamics simulations. The results show that the substrate in the glycosylated form is more stable and has a higher binding probability. This was attributed to the protection of the substrate binding pocket by Ser189 O-GlcNAc, which contributes to improved MDH1-substrate and substrate-substrate interactions. Further examination of the structural details revealed that the enhanced MDH1-substrate interaction was mainly due to the contact of Ser189 O-GlcNAc with protein residues Gln228, Gln229, and Arg98. This observation was confirmed by experiments analysing the activity of the corresponding triplet mutation (Q228A/Q229A/R98A). In addition, unlike the monomer, the MDH1 dimer did not show significant differences in substrate binding in the nonglycosylated and glycosylated forms. This may be due to the fact that the helical region involved in the substrate binding pocket is restricted by dimeric interfacial interactions and is not as flexible as in monomers, making it difficult for Ser189 O-GlcNAc to significantly affect it. Overall, the authors found that Ser189 O-GlcNAc could act as a molecular glue to enhance substrate binding and stability by stabilising the substrate binding pocket on the MDH1 monomer and improving protein-substrate and substrate-substrate interactions, which ultimately promotes the enzymatic activity of MDH1.
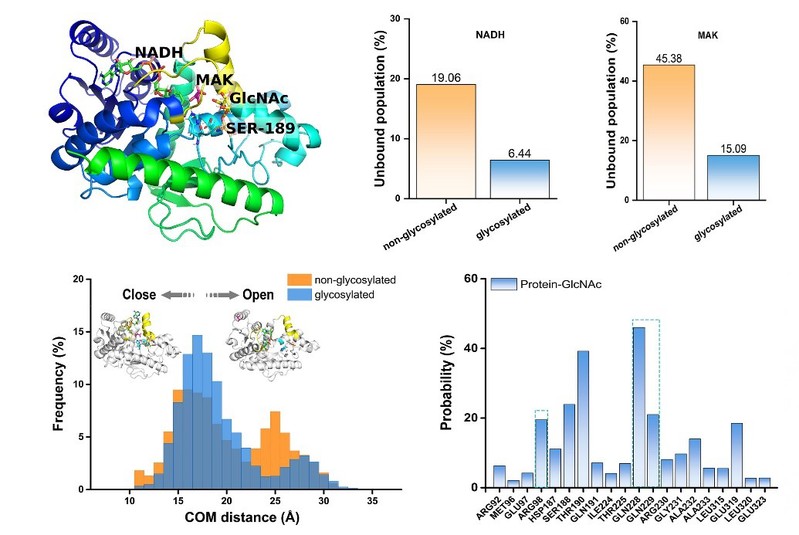
The initial conformation of monomeric pMDH1 in complex with the NADH and MAK (α-ketomalonic acid) substrates used in molecular dynamics simulations. The active site loop and helix are in yellow. The substrates, S189 glycosylation and the conserved His–Asp pair are shown in the stick. RMSD histogram showing the substrate stability of pMDH1 with S189 in both nonglycosylated and glycosylated forms. The NADH and MAK unbound population of the nonglycosylated and glycosylated S189 forms.
This study reveals the major functions of the OGT-MDH1 axis in the development and progression of PDAC. Given the high expression of MDH1 in PDAC and the positive correlation between MDH1 glycosylation levels and PDAC progression, the results highlight that MDH1 glycosylation intervention may be a potential strategy for the treatment of PDAC.
A working model of MDH1 O-GlcNAcylation in regulating Gln metabolism and tumor growth in PDAC.
The work lies in the intersection of glycochemical biology, tumour biology, and computational biology, and was supported by the National Key R&D Program of China, the National Science Foundation of China, the National Science Foundation of Zhejiang Province, National Independent Innovation Demonstration Zone Shanghai Zhangjiang Major Projects, Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study and the W. M. Keck Foundation. The article can be accessed at https://www.nature.com/articles/s41589-022-01085-5.

