Hydrogels are biocompatible, mechanically tunable, electrically conductive and optically transparent, making them ideal for tissue engineering, microlenses, ionic conductors, ionotronic devices, electroluminescent devices, soft robotic actuators etc. These applications rely on the stable and excellent mechanical properties of hydrogels under various conditions such as dehydration and low temperatures. However, network defects in hydrogels have a significant impact on their properties and often lead to degradation of their mechanical properties.
In nature, numerous organisms can survive against extreme conditions due to the strong hydrogen bonds formed by trehalose. Recently, Professor Shaoxing Qu and his colleagues from Zhejiang University introduced trehalose into hydrogels and found that trehalose can repair the network structure of a variety of hydrogels through covalent-like hydrogen bonding, which significantly improves their mechanical properties (including strength, tensile strength, and fracture toughness) over a wide range of temperatures while enabling them to tolerate extreme environments and side effects of desiccation. This strategy provides a versatile approach for the synthesis of extremely tolerant, high-strength, self-healing, and resilient hydrogels. The work was published in Science Advances and entitled A versatile hydrogel network-repairing strategy achieved by the covalent-like hydrogen bond interaction.
Hydrogel network-repairing strategy
After the addition of alginate to polyacrylamide (PAAm) hydrogels, strong hydrogen bonds formed between alginate molecules and many polar groups in the long chains of the polymer. The hydrogel network can be strengthened in several ways by the interaction of alginate with the hydrogel network.
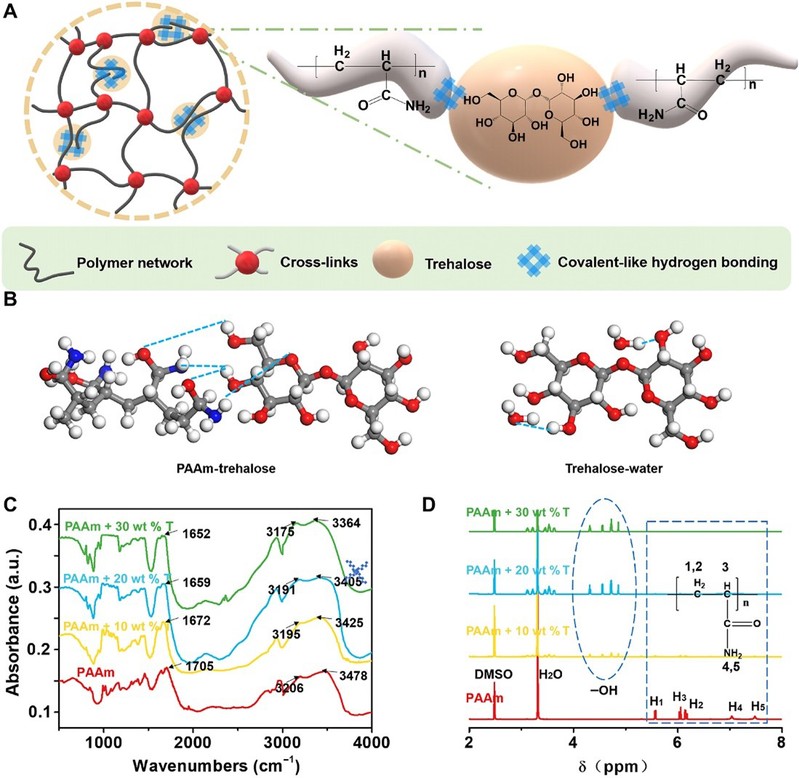
Figure 1 Repair strategies for trehalose networks through covalent hydrogen bonding.
Enhancement effect
Compared to pure PAAm hydrogels, the strength and tensile properties of hydrogels immersed in solutions containing different concentrations of algal sugars were significantly improved. They increased from 40 kPa and 1330% to 365 kPa and 2520%, respectively, with increasing algal sugar content because algal sugar can dynamically repair the hydrogel network through covalent-like hydrogen bonding.
The Irwin-Orowan model suggests that the fracture process of the hydrogel involves the breakage of a chain layer on the crack surface and the breakage of some polymer chains. Compared to the unmodified PAAm hydrogel, more energy is required to break one layer of polymer chains per unit area of the crack, and the mechanical dissipation near the crack tip is significantly higher due to the hydrogen bonds between the alginate and polymer chains forming additional 'polymer chains' in the crack plane. Finally, the fracture energy of the modified hydrogels increased significantly from 474 J/m2 to 5116 J/m2, as the fracture toughness of the hydrogels increased significantly with increasing alginate content.
The tensile properties of the algose-free PAAm hydrogels were very sensitive to defects. In contrast, the large cross-section PAAm hydrogels modified with trehalose remained highly ductile, even up to 41 times, and significantly improved the defect resistance of the hydrogels.
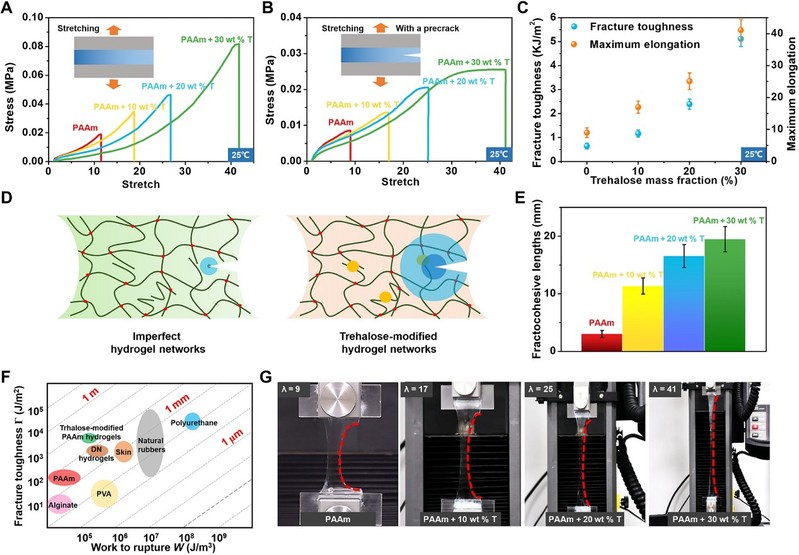
Figure 2 Improved of mechanical properties of PAAm hydrogels at room temperature by an trehalose network repair strategy
Tolerating low temperatures
At -15°C, the trehalose -free PAAm hydrogel completely freezes and becomes stiff and brittle, while the hydrogel modified with trehalose retains its high tensile strength and flexibility. This is because trehalose can disrupt the hydrogen bonding network of water, preventing the formation of ice crystals in hydrogels at low temperatures. As a result, the modified hydrogels remain partially frozen over a wide range of temperatures, i.e., small ice crystals are randomly distributed throughout the hydrogel, resulting in a final cracked, rough surface that is responsible for the increased toughness at low temperatures.
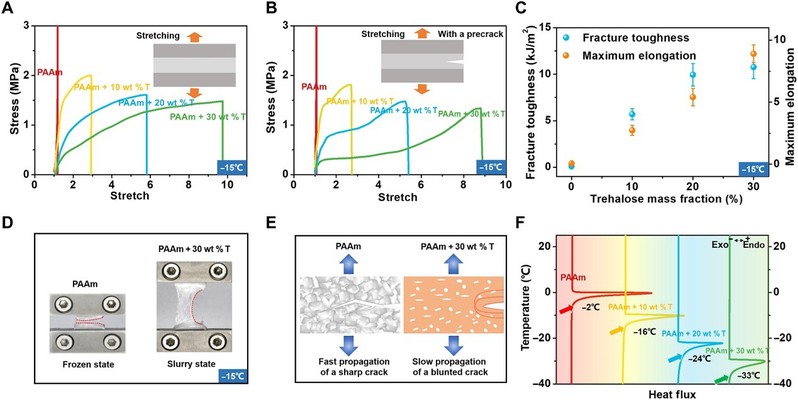
Figure 3 Trehalose -modified PAAm hydrogels maintain their hyperelasticity and flexibility at low temperatures
Tolerating dehydration
Trehalose can effectively slow down the water loss of hydrogels and protect the hydrogel network during and after dehydration. After freeze-drying at -80°C for 72 hours, all hydrogel samples had reduced volume and weight due to water loss and even disintegrated, became hard and brittle, and lost elasticity and function. In contrast, the hydrogels with 30 wt% alginate retained their super-elasticity and flexibility to stretch, fold and twist. This is mainly due to the support provided by the alginate clusters (smooth round or oval regions on the surface of the alginate-modified PAAm hydrogels) and the mechanism of water retention due to strong hydrogen bonding.
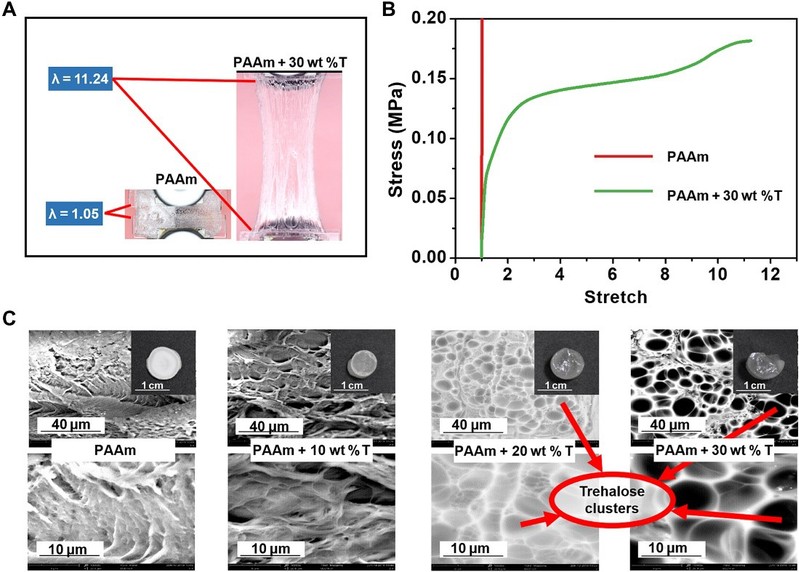
Figure 4 Trehalose-modified PAAm hydrogel maintain their mechanical properties after dehydration
General applicability
The network repair strategies of alginate and other sugars have been shown to be useful for a wide range of hydrogels. By soaking a hydrogel sample in an alginate solution, a network repair strategy can be easily implemented to impart self-healing properties to the hydrogel while improving its mechanical properties such as strength, tensile strength, initial shear modulus, and fracture energy.
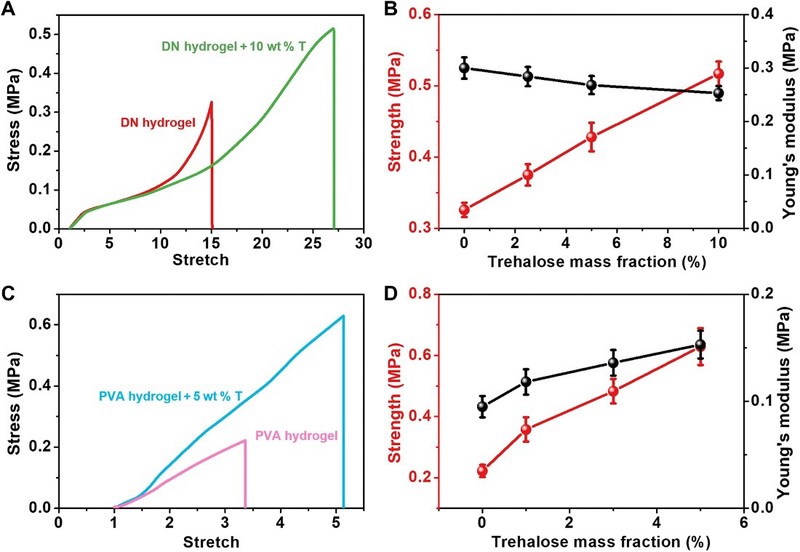
Figure 5 General applicability of threhalose network-repairing strategy
The comprehensive properties of the modified hydrogels expand their potential applications under various conditions. pAAm hydrogels have an electrical conductivity of about 0.32 S/m, which is no longer conductive at low temperatures. However, by incorporating threhalose, which mitigates the degradation of conductivity, the modified hydrogel maintains their conductivity at low temperatures through partial freezing.
The work also demonstrated a pressure/strain sensor that can operate at low temperatures. This requires a hydrogel with good electrical conductivity and excellent mechanical properties, which also maintain good stability and reversibility under cyclic loading, with stable changes in relative resistance, low residual strain and a large recovery ratio.
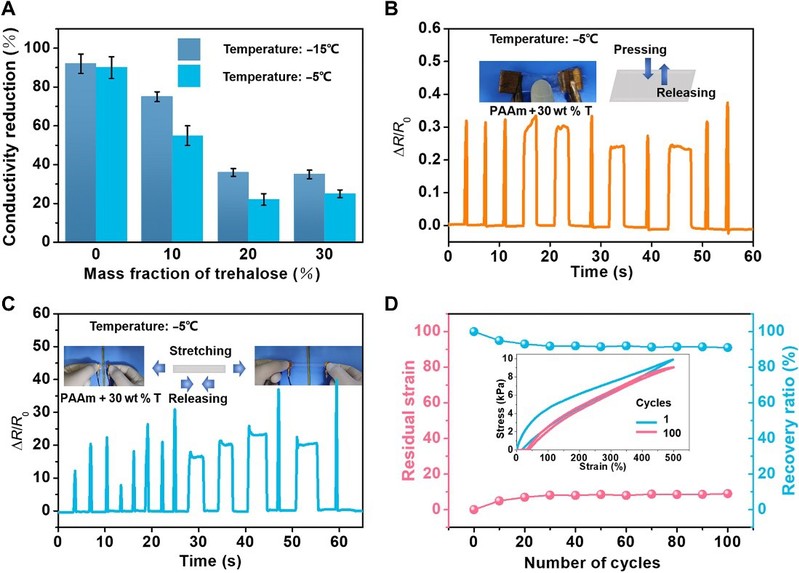
Figure 6 Applications are enabled by the stretchable, tough, anti-freezing, and anti-drying hydrogels
This work has shown that threhalose can act as an effective network repair agent for hydrogels by introducing covalent-like hydrogen bond interactions that aim to retain strength, strechability, fracture toughness and conductivity at low temperatures, expanding their potential applications in a variety of conditions.
To access the article, please visit https://www.science.org/doi/10.1126/sciadv.abl5066.
About Professor Qu
Dr. Shaoxing Qu is a Changjiang Chair Professor (from Ministry of Education, China) and Distinguished Young Scholar grant from National Natural Science Foundation of China. He serves as the Director of the Key Laboratory of Soft Machines and Smart Devices of Zhejiang Province, and is currently an Adjunct Professor of Shanghai Institute for Advanced Study of Zhejiang University (SIAS).
He got his bachelor degree from University of Science and Technology of China in1997, Master of Engineering from Tsinghua University in 2000, and Ph.D. from UIUC in 2004. After spending two years at Brown University as postdoc, he joined Zhejiang University in 2006 and was promoted to full professor in 2011. He has published more than 180 journalpapers.
Dr. Qu’s primary research interests include soft materials and soft machines, insect-scalerobots, mechanics of composites, and micro/nano mechanics.
About SIAS
Shanghai Institute for Advanced Study of Zhejiang University (SIAS) is a jointly launched new institution of research and development by Shanghai Municipal Government and Zhejiang University in June, 2020. The platform represents an intersection of technology and economic development, serving as a market leading trail blazer to cultivate a novel community for innovation amongst enterprises.
SIAS is seeking top talents working on the frontiers of computational sciences who can envision and actualize a research program that will bring out new solutions to areas include, but not limited to, Artificial Intelligence, Computational Biology, Computational Engineering and Fintech.

