Immune checkpoint blockade therapies, particularly PD-1/ PD-L1 inhibitors, extensively studies in recent years, have made important advances in oncology. However, due to individual heterogeneity and the high complexity of anti-tumor immunoregulation, currently approved immune checkpoint inhibitors are only effective in a small number of patients. In the vast majority of unselected solid tumors, the efficacy of PD-1 or PD-L1 inhibitors alone is only 10%-30%, and the corresponding therapies generally suffer from bottlenecks such as low response rates in some patients and limited treatment scenarios. Thorough deciphering and elucidation of the molecular regulatory mechanisms of relevant tumor immune checkpoints and tumor immune escape mechanisms may bring new hope for tumor patients.
Post-translational modification of proteins is one of the key cell biological events that determine their functional diversity and maintain the precision and complexity of regulation of cellular activities. As central nodal molecules of tumor immune defense, post-translational modifications such as glycosylation, ubiquitination, palmitoylation, acetylation and phosphorylation of PD -L1 and other immune checkpoints play important regulatory roles in their maturation, degradation and translocation. It is of great scientific importance and clinical relevance.

Recently, Professor Ruhong Zhou from Shanghai Institutes for Advanced Study, Zhejiang University, Professor Lin Aifu and Professor Zhou Tianhua from Zhejiang University, Professor Wang Wenqi from University of California, Irvine and Professor Li Xu from School of Life Sciences, Westlake University jointly published a paper in Nature Communications, entitled Promoting anti-tumor immunity by The paper, entitled Promoting anti-tumor immunity by targeting TMUB1, a modulator of PD-L1 polyubiquitination and glycosylation which reveals that targeting TMUB1, a regulator of PD-L1 post-translational modification, can effectively reverse the suppressive tumor-immune microenvironment and that the development of relevant immunotherapy combination targeting strategies has important clinical value.
Through in-depth analysis of clinical data, researchers found that key post-translational modification pathways such as glycosylation and ubiquitination were highly enriched in tumor patients with high PD-L1 expression and were significantly associated with low response rates to PD-1/PD-L1 monoclonal antibodies. The further finding of higher levels of PD-L1 post-translational modifications in tumor tissues suggests that insights into the regulatory mechanisms of PD-L1 post-translational modifications may provide potential targeting strategies for immunotherapies. Based on the above findings, the team combined mass spectrometry and biomarker analysis to successfully screen a series of PD-L1 candidate reciprocal molecules. Here, transmembrane and ubiquitin-like structural domain protein 1 (TMUB1), a quality control element of the endoplasmic reticulum membrane protein, was highly expressed in tumor tissues such as gastric and breast cancer and was strongly negatively correlated with CD8+ T cell infiltration, suggesting its potential regulatory function in PD-L1-mediated tumor immune escape. It has a potential regulatory function in PD-L1-mediated tumor immune escape. In-depth mechanistic studies confirmed that TMUB1 plays an important role in regulating several post-translational modifications of PD-L1. On the one hand, TMUB1 localized in the endoplasmic reticulum binds competitively with the E3 ubiquitin ligase HUWE1 to PD-L1, which strongly impairs the degradation of PD-L1 via the ubiquitination pathway and subsequently increases the protein stability of PD-L1; on the other hand, TMUB1 promotes PD-L1 glycosylation modification, maturation, cell membrane translocation and tumor microenvironment regulation by recruiting related glycosyltransferases. On the other hand, TMUB1 promotes PD-L1 glycosylation modification, maturation, cell membrane translocation, and tumor microenvironment regulation by recruiting related glycosyltransferases, ultimately leading to tumor immune escape.
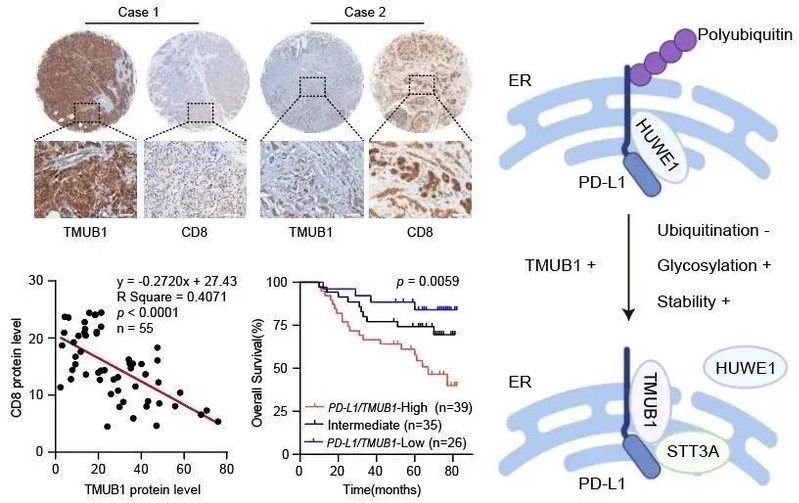
These results suggest the clinical potential of TMUB1 as a combined target for tumor immunotherapy. By leveraging the cross-domain strengths of multiple teams and through computational structural simulations, binding region screening, molecular design of peptide-like precursors, and functional validation in vitro and in vivo, researchers have developed a targeted peptide, PTPR, that successfully blocks the multiple post-translational modifications of TMUB1 to PD-L1 by inhibiting the binding of TMUB1 to PD-L1, and achieved significant results in various tumor models including gastric cancer and breast cancer. The peptide inhibitor has achieved significant inhibition of tumor growth in various tumor mouse models and also demonstrated the biological safety in vivo. In addition, the combination with immune checkpoint monoclonal antibodies such as αCTLA4 successfully reversed the tumor suppressive microenvironment, greatly enhanced the infiltration of cytotoxic T cells and the specific killing of tumor cells, and finally achieved tumor suppressive efficacy.

In conclusion, this study reveals the regulatory mechanism of post-translational modification of PD -L1 and the molecular mechanism of associated tumor immune escape, provides preclinical evidence that TMUB1 can be a reliable combination target for immunotherapy, and the development of a peptide-like precursor drug molecule demonstrates that targeting the molecular strategy of modulating the post-translational modification of PD -L1 has important clinical application value.
To access the article please visit Promoting anti-tumor immunity by targeting TMUB1 to modulate PD-L1 polyubiquitination and glycosylation | Nature Communications, including information on research design and source data. This work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study etc.
About Professor Ruhong Zhou
Ruhong Zhou, AAAS Fellow, APS Fellow, is currently Qiushi Chair Professor, Dean, College of Life Sciences; Dean, Shanghai Institute for Advanced Study; and Director, Institute of Quantitative Biology of Zhejiang University. He is also an Adjunct Professor at Department of Chemistry, Columbia University. Before that, he was a Distinguished Research Staff Scientist, and Head of the Soft Matter Science Department at IBM Research.
Professor Zhou’s research focus on Quantitative Biology; Machine Learning, Deep Learning in Biology; Biophysics; and Bio-Nano Interface (nanomedicine). He has authored and co-authored more than 300 journal publications with 25000+ total citations (Google H-index 81), filed 32 international patents and delivered 300+ invited talks at major conferences and universities worldwide. He is part of the IBM BlueGene team that won the 2009 National Medal on Technology (presented by President Obama). He has won the IBM Outstanding Technical Achievement Award (the highest technical award within IBM; 10 times), the IBM Outstanding Innovation Award (twice), and many IBM Research Division Awards. He also won the American Chemical Society DEC Award on Computational Chemistry. He was elected to AAAS Fellow (American Association of Advancement of Science) and APS Fellow (American Physical Society) in 2011. He received his PhD in Biophysical Chemistry from Columbia University, MS in Condensed Matter Physics and BS in Physics both from Zhejiang University.
About SIAS:
Shanghai Institute for Advanced Study of Zhejiang University (SIAS) is a jointly launched new institution of research and development by Shanghai Municipal Government and Zhejiang University in June, 2020. The platform represents an intersection of technology and economic development, serving as a market leading trail blazer to cultivate a novel community for innovation amongst enterprises.
SIAS is seeking top talents working on the frontiers of computational sciences who can envision and actualize a research program that will bring out new solutions to areas include, but not limited to, Artificial Intelligence, Computational Biology, Computational Engineering and Fintech.

