Metabolic chronic diseases such as obesity and diabetes, which are caused by the reduction of exercise and excessive food intake, has become more and more prevalent with the improvement of living standard and refined diet. Dietary intervention and healthy diet are one of the most effective strategies to prevent and control of chronic diseases. Resistant starch (RS) is a type of starch and degradation products do not get digested and absorbed in small intestines. As a new dietary fiber, RS enjoys important physiological functions in preventing and controlling diabetes, lowering blood lipids, controlling body weight and maintaining intestinal health. Rice is the main grain crop in China, but it contains low level of RS. Currently, little is known about the functional genes for RS synthesis, and it is challenging to breed high-RS rice varieties.
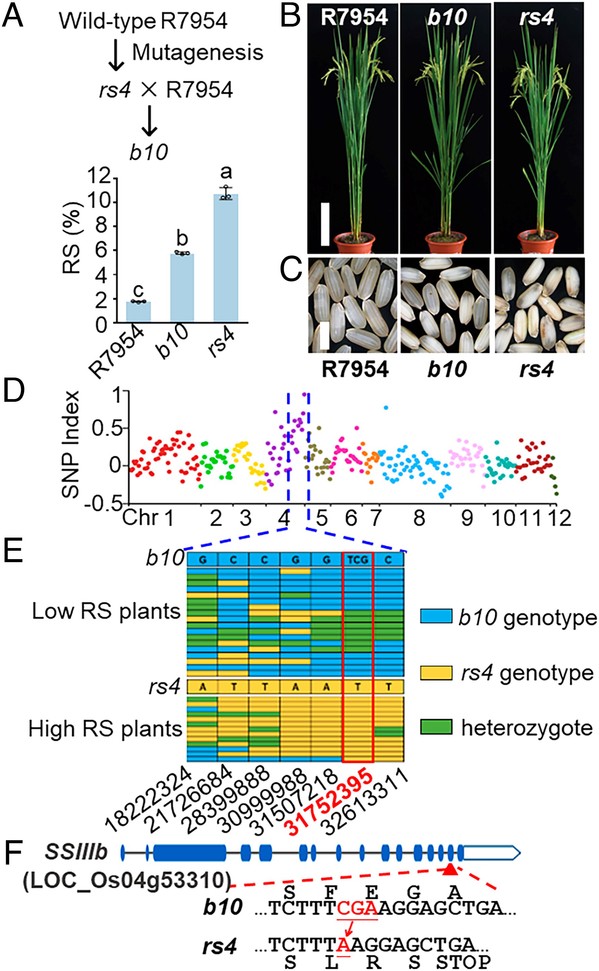
Characterization of the high-RS mutant rs4 and cloning of SSIIIb. (A) Pedigree and RS contents of wild-type R7954, high-RS mutant b10 and rs4. Values are means ± SD (n = 3), and different letters at top of each column indicate a significant difference at P < 0.05 determined by Tukey’s HSD test. (B) Plant morphologies of R7954, b10, and rs4, bar = 20 cm. (C) Seed morphologies of R7954, b10, and rs4, bar = 0.5 cm. (D) SNP index between extreme low- and high-RS pools in an F2 population generated from the cross between rs4 and b10. (E) Mapping SSIIIb with extreme low- and high-RS plants in high SNP index region. Blue box, the homozygous b10 genotype; yellow box, the homozygous rs4 genotype; green box, the heterozygous genotype. (F) Gene structure of SSIIIb and mutation sites in b10 and rs4.
Recently, Dianxing Wu, Professor from Zhejiang University and Adjunct Professor from Shanghai Institute for Advanced Study, Zhejiang University and collaborators had made progress in the study of the mechanism of RS synthesis in rice. They found that mutations in SSIIIa (Soluble starch synthase) could increase RS content in indica rice from less than 2% to 6% (Zhou et al., 2016) in earlier studies, and they further discovered that via resequencing and gene cloning, the higher level of RS in mutant rs4 (with 10.8% in cooked rice) obtained by physical mutagenesis was due to a common mutation in the SSIIIa and SSIIIb genes. SsIIIb mutants showed no significant change in RS content, but SsIIIaSsIIIb mutants showed a significant increase in RS content. The duplication of SSIII and neofunctionalization of SSIIIa with high expression levels in the endosperm was associated with the reduced RS contents in the cooked grains of tested cereals, whereas the dicots without this neofunctionalization of SSIII showed high RS contents in their cooked seeds.
The genetic study revealed that the loss-of-function SSIIIb and SSIIIa together with a strong Wx allele in the background collaboratively contributed to the high-RS phenotype of the rs4 mutant. The increased RS contents in ssIIIa and ssIIIa ssIIIb mutants were associated with the increased amylose and lipid contents. SSIIIb and SSIIIa proteins were functionally redundant, whereas SSIIIb mainly functioned in leaves and SSIIIa largely in endosperm owing to their divergent tissue-specific expression patterns.

Divergent functions of SSIIIa and SSIIIb in different tissues. (A) RS contents of R7954, rs4, Ubi:cSSIIIa/rs4, and SSIIIa:gSSIIIa/rs4. (B) Morphologies of seed and endosperm of R7954, rs4, Ubi:cSSIIIa/rs4, or SSIIIa:gSSIIIa/rs4. (C) RS contents of R7954, b10, SSIIIb:cSSIIIb/b10, and Ubi:cSSIIIb/b10. (D) Morphologies of seed and endosperm of R7954, b10, SSIIIb:cSSIIIb/b10, or Ubi:cSSIIIb/b10. (E) Total starch contents in leaves of ZH11, ssIIIaCR-1, ssIIIbCR-1, and ssIIIaCR ssIIIbCR-1 at the end of the day and night. (F) Starch levels in leaves of ZH11, ssIIIaCR-1, ssIIIbCR-1, and ssIIIaCR ssIIIbCR-1 detected by iodine staining at the end of the day and night. Bar = 0.1 cm. (G) Seed setting rate and (H) thousand-seed weight of ZH11, ssIIIaCR-1, ssIIIbCR-1, and ssIIIaCR ssIIIbCR-1. In A, C, and F, values are means ± SD (n = 3), in G and H, values are means ± SD (n = 10), and different letters at the top of each column indicate a significant difference at P < 0.05 determined by Tukey’s HSD test.
Furthermore, the authors found that SSIII experienced duplication in different cereals, of which one SSIII paralog was mainly expressed in leaves and another in the endosperm. SSII but not SSIV showed a similar evolutionary pattern to SSIII. The copies of endosperm-expressed SSIII and SSII were associated with high total starch contents and low RS levels in the seeds of tested cereals, compared with low starch contents and high RS levels in tested dicots.

SSII and SSIII homologous genes and their expression patterns and RS contents in different cereals and dicots. (A) Phylogenetic tree and expression patterns of the SSIII gene family. (Left) The phylogenetic tree of SSIII family of cereals including Oryza sativa, Triticum aestivum (B genome), Zea mays, and Setaria italica and dicots including Glycine max, Arabidopsis thaliana, and Solanum lycopersicum. (Right) Z-score normalized expression levels of SSIII genes were shown in the heatmap. (B) Phylogenetic tree and expression patterns of SSII gene family. (Left) The phylogenetic tree of SSII family of cereals and dicots as listed in A. (Right) Z-score normalized expression levels of SSII genes were shown in the heatmap. Gray boxes indicate that the gene expression was not detected (ND). (C) RS contents of total starch in the cooked seeds of five dicots including soybean, mung bean, almond, lemon, and sunflower and four cereals including rice, wheat, corn, and millet. (D) Total starch contents of nine species as listed in C. In C and D, values are means ± SD (n = 3) and different letters at the top of each column indicate a significant difference at P < 0.05 determined by Tukey’s HSD test.
RS potentially reduces the postprandial glucose and insulin responses to help control type 2 diabetes, obesity, and other related diseases. These findings shed light on the molecular mechanism of RS biosynthesis in rice, and provided critical genetic resources for breeding high-RS rice cultivars, and the evolutionary features of these genes may facilitate to generate high-RS varieties in different cereals.

Proposed model of SSIII duplication and reduced RS contents in cereals. SSII and SSIII were duplicated in cereals with the low RS content in seeds and the dicots contained high RS contents without neofunctionalization of SSII and SSIII. SSIIa and SSIIIa in cereals were preferentially expressed in the endosperm, whereas SSIIb, SSIIc, and SSIIIb in cereals and SSII and SSIII in dicots were preferentially expressed in leaves. To breed high-RS rice varieties, the deficiencies in SSIIIa and SSIIIb together could elevate both amylose and lipid contents to form amylose–lipid complex and increase the RS contents.
The work is published in Proc. Natl. Acad. Sci. USA and could be accessed at https://www.pnas.org/doi/10.1073/pnas.2220622120.
About Professor Dianxing Wu
Dr Wu graduated from Zhejiang University in 2001 and is the Head of Department of Applied Biosciences in College of Agriculture and Biotechnology, Zhejiang University. He is currently an Adjunct Professor of Shanghai Institute for Advanced Study of Zhejiang University (SIAS).
Dr Wu is interested in enhancing mutant rice germplasm high in functional components, special nutrients, environmental friendship and extreme-environment adaptation by establishing high-through put screening methodologies, elucidating the genetic basis and metabolite regulation of these mutated traits and breeding for new rice/plant varieties with the value-added functional, industry-specific and green ecological properties and efficient resource utilization, which will provide technical supports for biological industry from seed-oriental agriculture to food, pharmacy and chemistry.

