AMPylation is a posttranslational modification that generally modifies amino acid side chains of proteins with adenosine monophosphate (AMP). Dr Yongqun Zhu, Adjunct Professor of Shanghai Institute for Advanced Study, Zhejiang University, together with collaborators published the article “Legionella effector LnaB is a phosphoryl-AMPylase that impairs phosphosignalling” in Nature on May 22, 2024. They discovered a new class of LnaB phosphoryl-AMPylase effector proteins widely presented in pathogenic bacteria, and revealed a new way of phosphoryl-AMPylation modification and regulation of phosphorylation signaling, challenging the traditional concept that phosphorylation was terminal and cannot be further covalently modified in cell. This work also illustrated that pathogenic bacteria could protect the classical ubiquitin pathway and inhibit the immune defense response of the host through the same effector protein, so as to coexistence with host cells in short term.
Adenylation monophosphate modification (AMPylation) is a post-translational modification first discovered in 1967, and widely found in prokaryotes and eukaryotes, playing an important role in endoplasmic reticulum stress, neurodegenerative diseases, adaptive immunity etc. Previous studies have found that the modification was mainly catalyzed by the Fic et al. family of adenosine monophosphate lyase (AMPylase), which transfers AMP groups to target proteins. Previously identified AMPylation sites include threonine, tyrosine, serine, and other residues of the protein substrate.
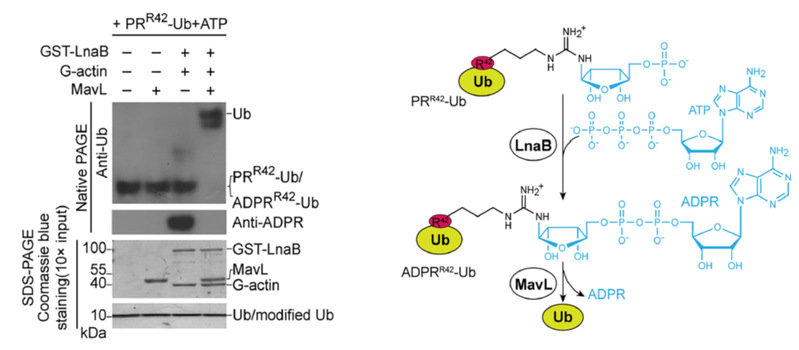
Legionella pneumophila causes Legionnaires' disease, and multiplies by infecting lung macrophages and forming distinctive membrane vesicle within host cells. In 2016, several studies discovered that Legionella pneumophila catalyzed an atypical ubiquitylation process independent of E1 and E2 via SidE family effectors. Atypical ubiquitination process and subsequent effector DupA/B promoted the maturation of membrane vesicles in Legionella pneumophila through an intermediate step of ADP-ribosylation modification (ADPRR42-Ub) to ubiquitin (Ub) arginine residue 42, which ultimately covalently attached ubiquitin as a phosphoribosylation (P-ribose) to a serine residue in a related substrate. Phosphoribosylated ubiquitin molecules (PRR42-Ub) are cytotoxic, suggesting that special effector may be required to eliminate them or to modify ubiquitin to facilitate Legionella pneumophila survival nd short-term coexistence in host cell.
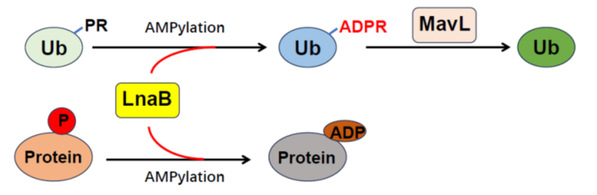
To verify the hypothesis above, the authors established a unique in vitro and in vivo screening system to reveal effector protein LnaB, a unique AMPylase (adenylase) that leveraged ATP as ligand and host actin as activator to generate ADPRR42-Ub by phosphoryl-catalyzed monophosphorylated adenylation of PRR42-UB. This cascade reaction, catalyzed by LnaB and MavL, reversed the atypical ubiquitination process, thereby protecting ubiquitination pathway essential for both intracellular survival and physiological processes in Legionella pneumophila.
The study further revealed that LnaB was able to inhibit yeast growth, and since PRR42-Ub is produced by SidE family effectors instead of proteins in yeast, the authors believed that there may be substrates for LnaB in cell. LnaB overexpression experiments produced a large number of adenylation monophosphate modification signals in 293T cells. LnaB could affect PRR42-Ub phosphoryl group widely presented in all phosphorylated proteins, suggesting LnaB may modify phosphorylated proteins.
Subsequent experiments revealed that overexpression of a persistently activated form of tyrosine kinase NPM-ALK or the addition of the phosphatase inhibitor okadaic acid treatment significantly increased the level of LnaB-catalyzed AMPylation. Via mass spectrometry, LnaB was found to phosphorylate adenosine monophosphate, and thus produce specific ADPylation modifications on peptide substrates containing phosphorylated serine, phosphorylated threonine, and phosphorylated tyrosine residues.
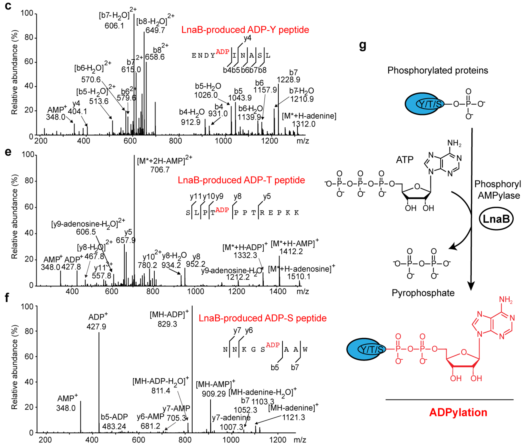
In this study, LnaB was found to phosphorylate conserved phosphotyrosine residues on the activation loop of Src family kinases, which are essential during infection. The modified Src family kinases were inactivated, inhibiting downstream phosphorylation signaling and subsequent mediation to immune signaling pathways. LnaB is the only bacterial virulence factor identified to date that can directly modify phosphorylated tyrosine residues in activating Src family kinases.
A homology search in this study subsequently identified 162 LnaB homologous proteins widely distributed in more than 20 genera, which constituted a novel family of phosphorylated adenosine monophosphate adenylases. The family has two conserved SG and H-E catalytic motifs, which differ from the previously discovered structure of the Fic family with a novel folding pattern. Structural studies showed that actin binded to the C-terminus of LnaB, stabilizing the β hairpin structure in catalytic domain, and ensured the binding of ligand ATP, activating the enzymatic activity of LnaB. 161 family members have signal peptides secreted from type III, type IV, or type VI systems, suggesting they may act on the corresponding target cells, with only 1 member as exception, which may act on the bacteria themselves.
The significance of this work include:
1. a new family of phosphoryl-AMPylases with unique catalytic motifs and structural features was discovered;
2. discovering of adenylation (AMPylation) modification targeting phosphoryl group, which illustrated AMPylation occurs beyond amino acid residues;
3. discovered a novel way to regulate phosphorylation signaling, breaking the traditional concept that phosphorylation was terminal and cannot be further covalently modified in the cell;
4. defined a novel post-translational modification of proteins, namely ADPylation modification;
5. revealed that pathogenic bacteria used the same effector to protect the ubiquitin pathway of the host and inhibit its immune defense for short-term coexistence;
6. The discovery of LnaB family revealed a conserved mechanism for regulating the host signaling pathway in pathogenic bacteria, providing new ideas for the study of pathogenicity and effector in pathogenic bacteria.
The ADPylation modification identified in this study extended the understanding of protein post-translational modification and demonstrated the feasibility of phosphorylating protein phosphoryl group modifications, providing unpreceded regulatory mechanisms for protein function and signal transduction. Since Src family kinases play important roles in a variety of human diseases, exploitation of their unique activity could potentially be applied to diseases treatment. Further studies on the function of LnaB family effector will open up new possibilities in the study of pathogenic bacterial effector.
To access the article please visit https://www.nature.com/articles/s41586-024-07573-z.

