Alzheimer's disease (AD) is a complex, multifactorial neurodegenerative disorder with an insidious onset. At molecular level, the deposition of β-amyloid (Aβ), produced by the processing of amyloid precursor proteins due to genetic susceptibility and environmental factors etc, is considered as one of the main pathological features of AD. In recent years, the amyloid cascade hypothesis (ACH) has been widely validated, and a key focus has been the role of Aβ oligomers as triggers for neurological inflammation and toxicity, a critical aspect in drug development. However, developing drugs targeting Aβ is extremely challenging since Aβ peptides are intrinsically disordered proteins and there is no explicit targets. Currently, only one antibody drug targeting Aβ, Lecanemab(Leqembi), has been approved. However, due to factors such as the short half-life and high cost of biologics, there is a shortage of such drug candidates.
Recently, Prof. Ruhong Zhou from Shanghai Institute for Advanced Study, Zhejiang University, Prof. Ning Chen from the Department of Materials and Chemistry at Soochow University, and Associate Prof. Zaixing Yang from the Institute of Radiology and Interdisciplinary Research at the School of Medicine, Soochow University, published a work in ACS Nano titled Rational Design of Dual-Functionalized Gd@C82 Nanoparticles to Relieve Neuronal Cytotoxicity in Alzheimer's Disease via Inhibition of Aβ Aggregation. They successfully designed and prepared a multifunctional nanoparticle inhibitor, f-Gd@C82, targeting the driving forces behind Aβ aggregation and the spatial structure of Aβ amyloid fibrils. f-Gd@C82 not only efficiently redirects the self-assembly of Aβ peptides and inhibits the early growth of protofibrils, but also breaks down oligomers, highly ordered protofibrils, and even mature Aβ fibrils. This approach could effectively alleviate the neuronal cytotoxicity caused by Aβ.
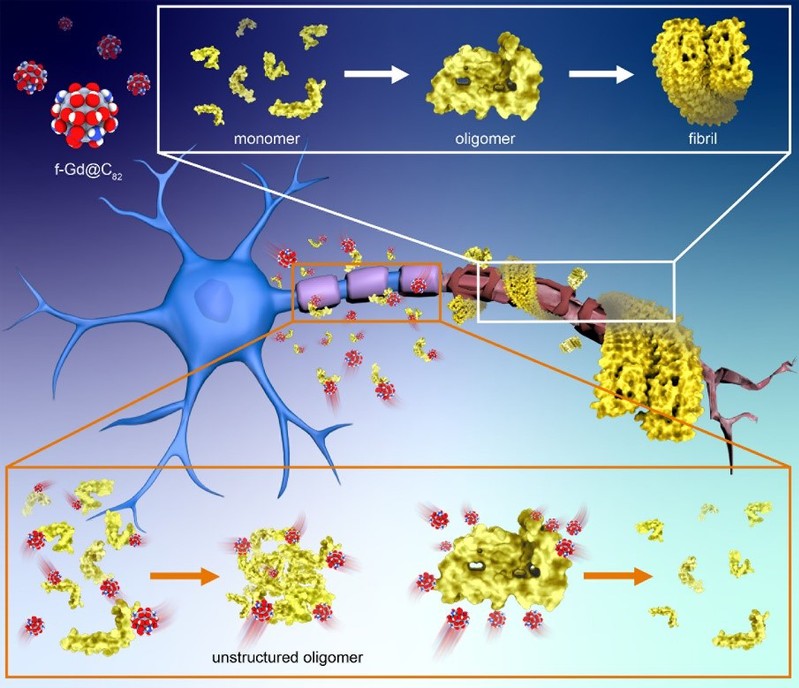
The authors have worked on Aβ peptide aggregation dynamics and its molecular mechanisms for a long time. Previous studies found that electrostatic, van der Waals, and hydrophobic interactions between the side chains of Aβ peptides drive the initial polymerization of peptide chains. Mature Aβ amyloid fibrils are formed by multiple layers of β-sheets arranged in crossed β-ridges, and the internal side chains of adjacent β-sheets are highly interlocked in a zipper-like manner, creating a dry hydrophobic interface. Such highly ordered and compactly stacked structure makes mature Aβ amyloid fibrils extremely thermodynamically stable. The microphysicochemical structure of Aβ amyloid fibrils provides key insights for rational design of drugs for AD prevention or treatment, e.g., introducing disordered interactions (chaotic) to block early Aβ aggregation or to break down the highly ordered protofibrils preformed, or even mature protofibrils. In this work, the authors choose an embedded metal fullerene (Gd@C82) as a framework for the functionalization design. Firstly, the metal-embedded structure is highly stable and easy to modify. Second, the high specific surface area enables itself to be modified by a large number of functional groups. In addition, the relatively rigid, spherical structure inherits a site-blocking effect to prevent peptides tightly binding together. The biocompatibility of Gd@C82 analogs has also been evaluated in a variety of biomedical scenarios, such as antitumor therapy.
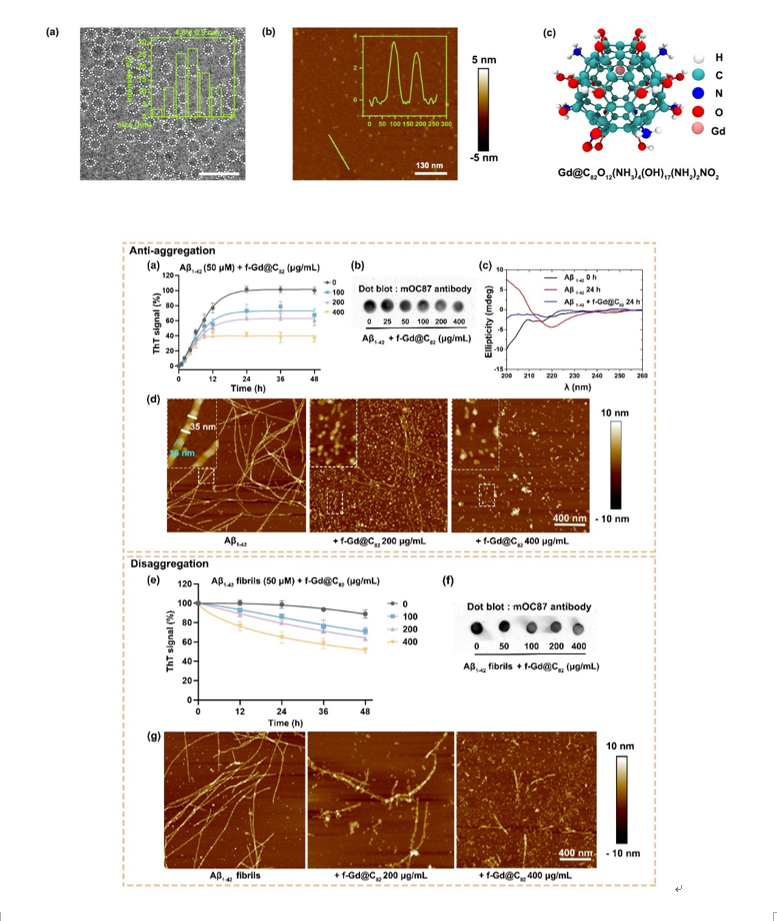
Previous studies have shown that hydrophobic drug molecules could inhibit Aβ peptide aggregation and disrupt the structure of mature amyloid fibrils through hydrophobic interactions with hydrophobic fragments. However, these molecules often suffer from poor dispersion, low bioavailability, and complex toxicological effects. Here the authors introduced a large number of disordered hydrogen bonding sites and charged groups to the surface of Gd@C82. This functionalization increased solubility, dispersibility, and biocompatibility of Gd@C82 nanoparticles (f-Gd@C82 NPs). The introduced unsaturated hydrogen-bonding sites could form a disordered hydrogen-bonding network with the peptide backbone, counteracting the formation of an ordered hydrogen-bonding network between the backbones of the Aβ peptide chain. The synthesized f-Gd@C82 NPs are approximately 5 nm in size. Several molecular biology experiments have demonstrated that f-Gd@C82 NPs could efficiently induce Aβ1-42 peptide chains to form disordered structures rather than highly ordered β-sheet structures. Furthermore, these nanoparticles could efficiently decompose protofibrils, and break down highly ordered protofibrils into disorganized, low-toxicity oligomers with a scattered distribution, thus significantly reducing their neuronal toxicity.
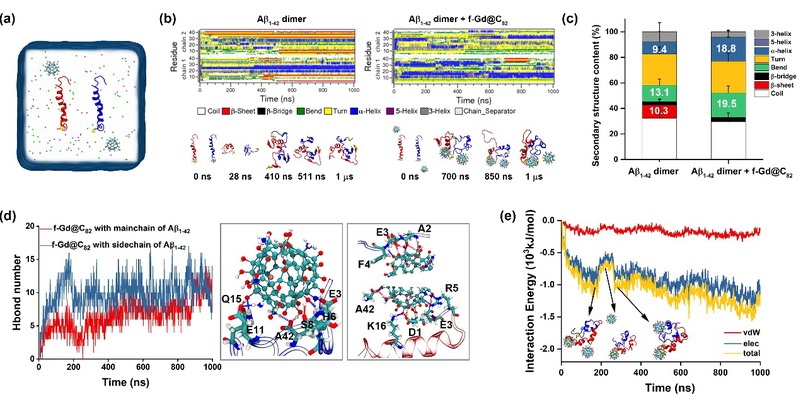
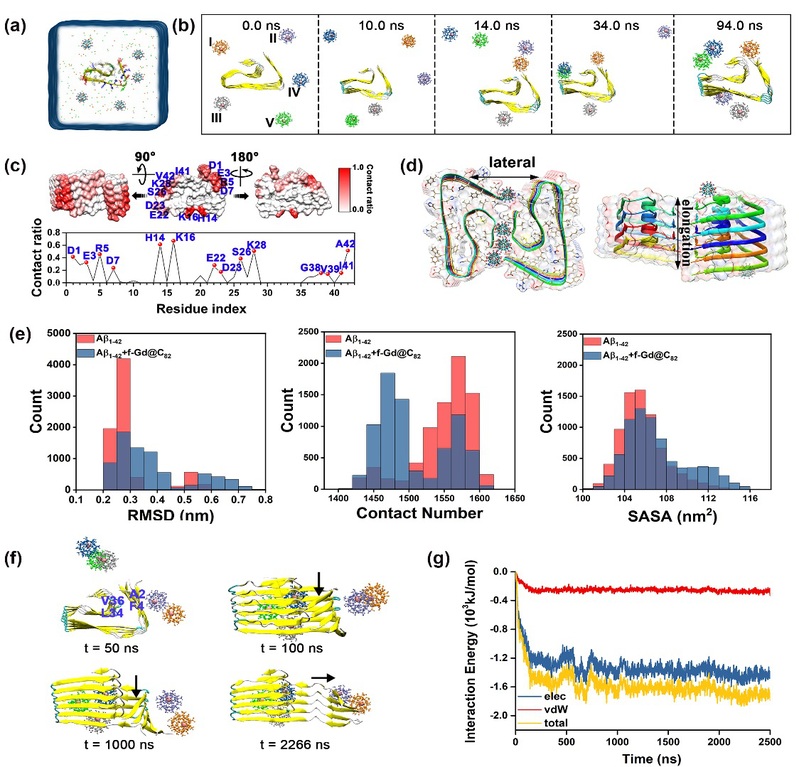
 The authors further explored the molecular mechanism of how f-Gd@C82 NPs inhibit Aβ aggregation via all-atom molecular dynamics (MD) simulations. The results showed that in the absence of f-Gd@C82 NPs, even Aβ1-42 dimers could rapidly self-assemble into a β-fold structure. However, when f-Gd@C82 NPs were present, the formation was significantly inhibited. f-Gd@C82 NPs formed a highly stable, disordered hydrogen-bonding network with all accessible hydrogen-bonding sites on the peptide chain, competitively inhibiting the formation of a continuous and ordered hydrogen-bonding network. Additionally, electrostatic interactions and spatial site-blocking synergistically inhibited the aggregation of Aβ1-42 peptide. f-Gd@C82 NPs were rapidly adsorbed onto the surface of Aβ1-42 protofibril by specifically binding to the charged residues, forming a hydrogen-bonding network with both the side chaina and main chains of protofibril. Such binding hindered the growth of protofibril. Furthermore, f-Gd@C82 NPs interacted with the N-terminus of Aβ1-42 protofibrils, disrupting the Ala2-Phe4-Leu34-Val36 hydrophobic core, leading to the misalignment and opening of N-terminal fibers.
The authors further explored the molecular mechanism of how f-Gd@C82 NPs inhibit Aβ aggregation via all-atom molecular dynamics (MD) simulations. The results showed that in the absence of f-Gd@C82 NPs, even Aβ1-42 dimers could rapidly self-assemble into a β-fold structure. However, when f-Gd@C82 NPs were present, the formation was significantly inhibited. f-Gd@C82 NPs formed a highly stable, disordered hydrogen-bonding network with all accessible hydrogen-bonding sites on the peptide chain, competitively inhibiting the formation of a continuous and ordered hydrogen-bonding network. Additionally, electrostatic interactions and spatial site-blocking synergistically inhibited the aggregation of Aβ1-42 peptide. f-Gd@C82 NPs were rapidly adsorbed onto the surface of Aβ1-42 protofibril by specifically binding to the charged residues, forming a hydrogen-bonding network with both the side chaina and main chains of protofibril. Such binding hindered the growth of protofibril. Furthermore, f-Gd@C82 NPs interacted with the N-terminus of Aβ1-42 protofibrils, disrupting the Ala2-Phe4-Leu34-Val36 hydrophobic core, leading to the misalignment and opening of N-terminal fibers.
f-Gd@C82 NPs can redirect peptide self-assembly into disordered, non-toxic aggregates, impede the early growth of protofibrils, and disassemble oligomers. The authors further investigated whether these nanoparticles could effectively intervene in Aβ1-42-induced AD. Soluble ordered Aβ1-42 oligomers (oAβ1-42) are most neurotoxic to neurons, and the authors examined the neurotoxicity of oAβ1-42 in the presence of f-Gd@C82 NPs. The model showed that f-Gd@C82 NPs significantly reduced oAβ1-42-induced neuronal death and synaptic damage.
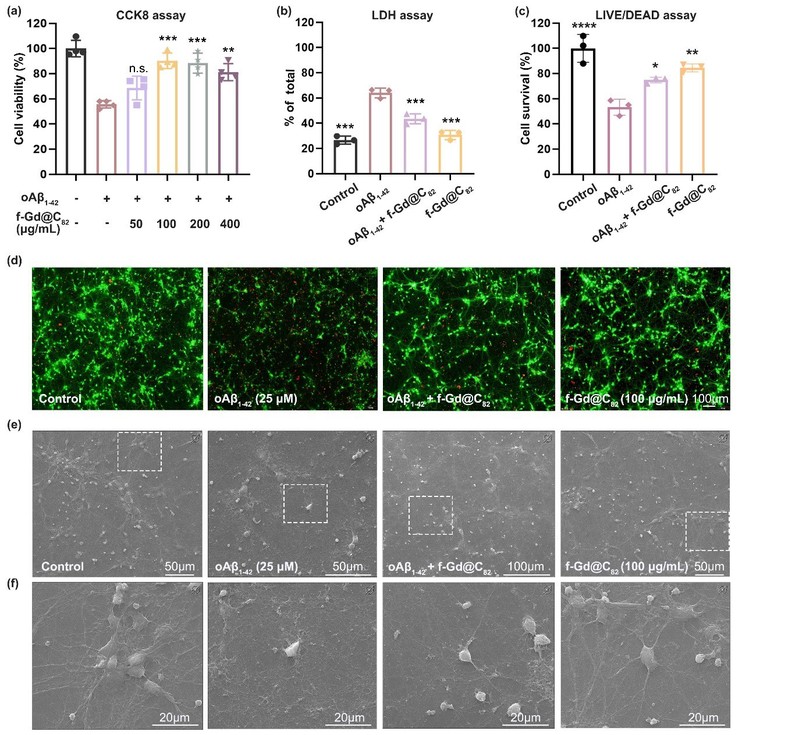
Notably, f-Gd@C82 NPs addressed the problems of GO-based nanosheets and other multifunctional nanoinhibitors such as g-C3N4. For instance, while the highly hydrophobic nature of GO nanosheets inhibit peptide aggregation and decompose mature protofibrils, it also results in poor dispersion in water and acute toxicity to cell membrane. Additionally, NIR beams cannot reach tissues in deep brain, limiting the effectiveness of NIR-triggered GO nanosheets and g-C3N4 surfaces in decomposing mature protofibrils. High temperatures may pose further risks to patients. The hydrophilic f-Gd@C82 NPs designed and developed in this work mitigate the assembly of Aβ aggregation by maximizing disorder, thereby reducing neuronal cytotoxicity without any external stimuli. This approach may provide a new direction for designing anti-aggregation-based AD management strategies.
The work is at the interaction of neurobiology, nanomedicine and computational biology, and is supported by the National Key R&D Program of China, the grants from the National Natural Science Foundation of China, the National Independent Innovation Demonstration Zone Shanghai Zhangjiang Major Projects and the Starry Night Science Fund. To access the work please visit https://pubs.acs.org/doi/10.1021/acsnano.3c08823.

