The bacterial flagellum is a proteinaceous organelle responsible for motility, which is crucial for bacterial infection, biofilm formation and survival in various environments. The flagellum is made up of three distinct parts: the motor, the hook and the filament. The motor is a bidirectional rotary nanomachine that provides the power for the rotation of the flagellum. However, the C ring structure is highly dynamic and easily dissociates from the flagellar motor during endogenous purification, making the study of the structural mechanism of the flagellar motor rotation direction regulation difficult. As a result, the molecular mechanism of the rotation direction conversion of bacterial flagellar motors has been unclear for a long time, and it is one of the important long-standing unresolved issues in the field.
Recently, Dr Yongqun Zhu, Professor of Zhejiang University, Adjunct Professor of Shanghai Institute for Advanced Study, Zhejiang University, together with collaborators, published an article "Structural Basis of the Bacterial Flagellar Motor Rotational Switching" in Cell Research. By constructing an activated mutant of the chemokine protein CheY and purifying endogenous flagellar motor particles containing the cytoplasmic ring (C ring) of the direction switch complex from the pathogen Salmonella Typhimurium, two high-resolution cryo-electron microscopy structures of the complete flagellar motor-adapter apparatus complex in counterclockwise and clockwise rotation directions were resolved, which clearly revealed that CheY protein binding leads to conformational changes in the direction switch complex, and proposed the concept of inner membrane repositioning of the stator unit of the flagellar motor, thereby clarifying the molecular mechanism of rotation direction conversion of bacterial flagellar motors, correcting all previous misunderstandings about the assembly and structure of C ring, and showing people the structure and working principle of the complete bacterial flagellar motor.
The authors had been studying the working mechanism of bacterial flagellar motors and continuously optimizing the purification system of endogenous flagellar motors of Salmonella. By constructing an activated CheY mutant (CheY**, CheYD13K&Y106W mutant), they successfully obtained the complete flagellar motor-connector complex particles of Salmonella containing C ring in counterclockwise and clockwise rotation states, and resolved their cryo-EM structures (Figure 1). The flagellar motor-connector complex structure containing CCW-C ring contains a total of 341 subunits, composed of 15 different flagellar proteins, with an overall molecular weight of about 10.2 MDa and a height of about 680 Å. The entire model of the flagellar motor-connector complex containing CheY**-bound CW-C ring is composed of 375 subunits and is about 664 Å in height. The C ring in both rotational states has C34 symmetry, and is composed of 34 FliG, 34 FliM and 102 FliN in a molar ratio of 1:1:3. It has extensive and unique cross-interactions between subunits, and can be divided into four subrings: the inner subring, the upper subring, the middle subring and the bottom subring. Among them, FliG stacks to form an upper subring structure that can interact with the stator unit by arranging its C-terminal domain FliGCC. The binding of CheY** causes the overall structure of the C ring to tilt significantly and shrink inward, and to approach the MS ring and the bacterial inner membrane by 16 Å (Figure 1).
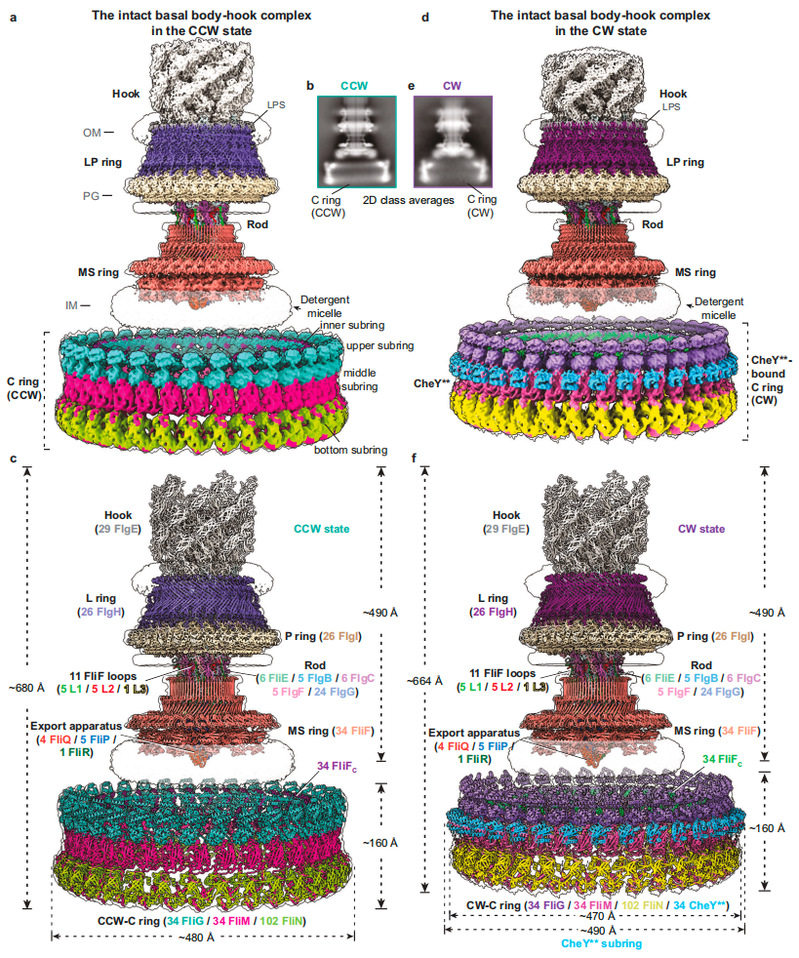
Figure 1. Overall structures of the C ring-containing basal body–hook complex in the CCW and CW states.
The authors found that the activated CheY bind to the gap formed by the outer surface of the adjacent FliMM and FliGM domains through the N-terminal domain of FliM, causing the various structural units of the C ring to undergo conformational changes to varying degrees, especially the conformation of the FliG subunit had undergone a huge change (Figure 2). The binding of CheY changes the spatial position of all the domains of the FliG subunit and the relative distance between the domains, which in turn causes the FliGCC domain to rotate 180° and move 10 Å toward the center of the motor, causing the core helical structure αtorque of the FliGCC domain that interacts with the stator unit to flip its orientation and electrostatic potential energy distribution. The conformational change of the C ring indicates that the stator unit must move toward the center of the motor and needs to be repositioned on the bacterial inner membrane to adapt to the change in the spatial position of αtorque. By analyzing the previously reported cryo-tomography data and establishing a complete flagellar motor structure model containing the stator unit (Figure 3), it was proved that the stator unit is indeed repositioned on the bacterial inner membrane.
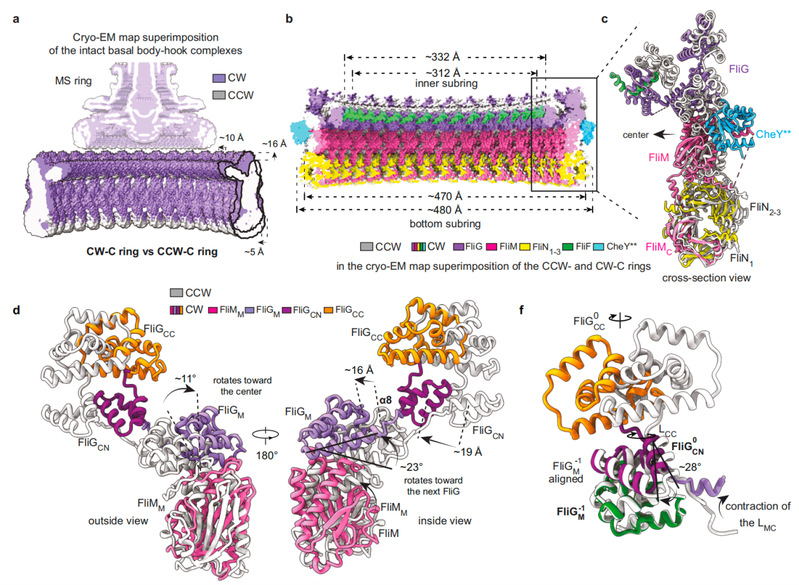
Figure 2. CheY**-induced conformational changes of the C ring in the intact motor.
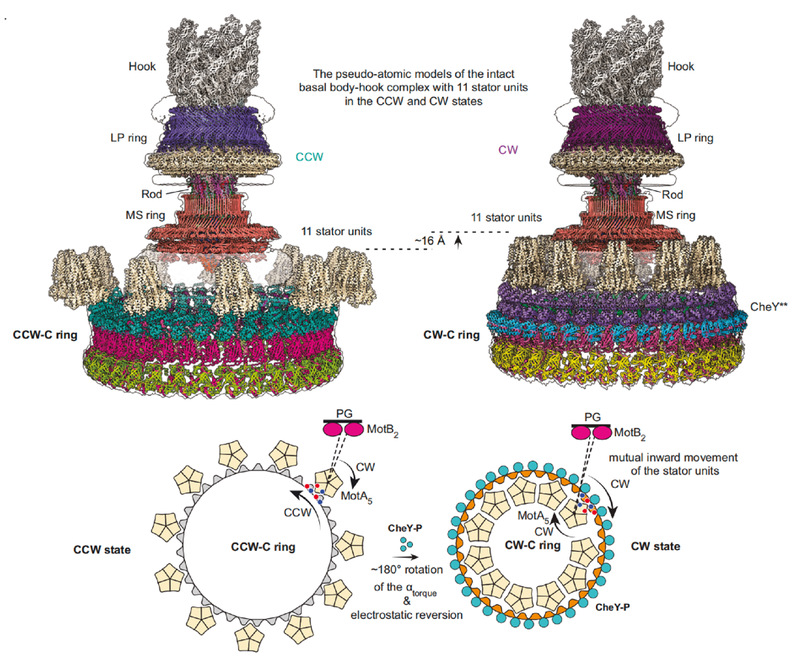
Figure 3. The proposed rotational switching mechanism of the bacterial flagellar motor.
The above data analysis shows the molecular mechanism of the flagellar motor direction conversion: when there is no CheY protein binding, the stator unit rotating in the clockwise direction is located on the outside of the FliGCC subring of the C ring, driving the flagellar motor to rotate in the counterclockwise direction. When CheY binds to the C ring, it promotes the conformational change of the C ring, shrinks toward the center of the flagellar motor and moves upward as a whole, thereby causing the conformational change of the FliGCC domain, and finally causing the αtorque on the FliGCC to flip 180°, thereby realizing the stator unit from the outside of FliG to the inside, and spatial repositioning on the inner membrane, prompting the C ring to rotate in the clockwise direction, thereby realizing the conversion of the flagellar motor direction (Figure 3).
This work challenged the previous concept that the stator was completely fixed on the bacterial inner membrane, refuted previous hypotheses that the direction change of flagellar motor was due to the structural extension of C ring, and showed the complete three-dimensional structure and working mechanism of flagellar motor, laying a theoretical foundation for the design of new antibacterial drugs and new rotary nanorobots. To access it please visit https://www.nature.com/articles/s41422-024-01017-z.

